Investigation of dioxygen activation by copper(ii)–iminate/aminate complexes†
Abstract
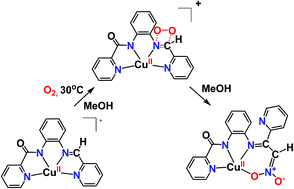
The activation of dioxygen by metal ions is critical in chemical and bio-chemical processes. A scientific challenge is the elucidation of the activation site of dioxygen in some copper metalloproteins, which is either the metal center or the substrate. In an effort to address this challenge, we prepared a series of new copper(II) complexes (1·2H2O, 2·CH3OH, 3) with bio-inspired amidate ligands and investigated their activity towards dioxygen activation. The secondary amine group ligated to copper(II) of the complex 1·2H2O in methyl alcohol is oxidized (2e−) by air dioxygen in a stepwise fashion to an imine group, affording complex 2. The copper(II) complex 2 in methyl alcohol induces the 4e− oxidation by air dioxygen of the imine functionality ligated to copper(II) to an azinate group, resulting in the isolation of a dinuclear azinate copper(II) compound (4). Experimental and computational studies, including X-band c. w. EPR, UV-vis and ESI-MS spectroscopy and density functional theory computations, indicate a direct attack of the dioxygen on the –HC![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N– group ligated to copper(II), and a possible mechanism of the oxidation of the –HC
N– group ligated to copper(II), and a possible mechanism of the oxidation of the –HC![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N– functionality ligated to copper(II) to an azinate group is provided. This unprecedented activation of dioxygen by a copper substrate paves the way for further exploration of the O2 activation mechanisms in enzymes and the development of effective catalysts in O2-involved green organic synthesis.
N– functionality ligated to copper(II) to an azinate group is provided. This unprecedented activation of dioxygen by a copper substrate paves the way for further exploration of the O2 activation mechanisms in enzymes and the development of effective catalysts in O2-involved green organic synthesis.


 Please wait while we load your content...
Please wait while we load your content...