A palladacyclic N-heterocyclic carbene system used to probe the donating abilities of monoanionic chelators†
Abstract
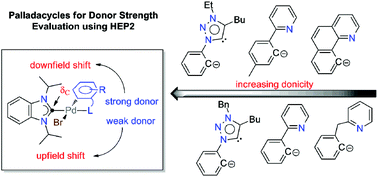
A series of NHC-containing [C^N]- or [C^C′]-type palladacyclic complexes of the general formula [PdBr(iPr2-bimy)(L^X)] (5–8, 11, 12, iPr2-bimy = 1,3-diisopropylbenzimidazolin-2-ylidene) have been synthesized and fully characterized. Using these complexes, the donating abilities of monoanionic chelators were probed for the first time. The [C^N]-type palladacycles 5–8 were prepared from acetato-bridged dipalladium complexes [Pd(μ-CH3COO)(C^N)]2 (1–4) and iPr2-bimy·H+Br−as precursors. In the case of the [C^C′]-type NHC palladacycles (11, 12), the hetero-bis(NHC) complexes trans-[PdBr2(iPr2-bimy)(trz)] (8, 9, trz = 1,2,3-triazolin-5-ylidene) containing the iPr2-bimy probe were first prepared followed by acetate-assisted cyclopalladations. The 13Ccarbene NMR signals of the iPr2-bimy ligands in all complexes (i.e. HEP and HEP2 values) are found to rationally reflect the donating abilities of the incorporated trz or [L^X]-type chelators with the exception of the Bzpy ligand (Bzpy = 2-(2-pyridinylmethyl)phenyl-C,N). This has been attributed to its larger bite angle, the resulting varied coordination geometry and the lack of electronic delocalization between the two donor units. The donicities of [L^X]-type chelators studied in this work were found to surpass those of all other bidentate ligands evaluated by HEP2 thus far.


 Please wait while we load your content...
Please wait while we load your content...