Synthesis, characterization and photocatalytic activity of mixed-metal oxides derived from NiCoFe ternary layered double hydroxides
Abstract
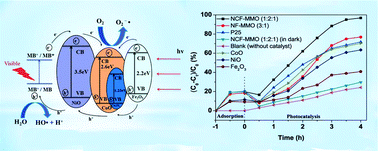
Ternary NiCoFe mixed-metal oxides (NCF-MMOs) with different Ni/Co/Fe ratios were successfully synthesized through a hydrotalcite-like precursor route by co-precipitation of appropriate amounts of metal salts from homogeneous solution, followed by calcination at 600 °C. X-ray diffraction (XRD) patterns revealed the formation of well crystalline layered double hydroxides (LDHs), particularly at the M2+/M3+ ratio of 3 : 1. Brunauer–Emmett–Teller (BET) analysis revealed that the resulting NiCoFe LDHs possessed large specific surface areas (66.9–93.8 m2 g−1). The NCF-MMO (1 : 2 : 1) samples were demonstrated to be formed by the aggregation of regular cubes with an edge length of about 2 μm, and each cube was accumulated with many fine particles with a size of ∼130 nm. UV–vis diffuse reflection spectroscopy (DRS) confirmed that the samples showed a broad absorption in the visible-light region (450–750 nm), with a low band gap of 2.33–2.77 eV. The calcined samples with a Ni/Co/Fe molar ratio of 1 : 2 : 1 possessed the best photocatalytic activity with 96.8% degradation of methylene blue (MB) dye under visible light irradiation for 4 h, which exceeded those of commercial P25 TiO2, binary NiFe mixed-metal oxides and pure Fe2O3, CoO and NiO particles under the same conditions. NCF-MMO (1 : 2 : 1) also had a strong degradation effect on the non-dye pollutant phenol as well. Kinetic studies suggested that the degradation of MB followed a pseudo-first-order kinetic behavior. The photodegradation mechanism of NCF-MMOs was also discussed.


 Please wait while we load your content...
Please wait while we load your content...