Synthesis, characterization and application of organorhenium(vii) trioxides in metathesis reactions and epoxidation catalysis†
Abstract
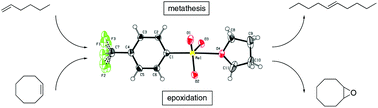
Four novel organorhenium(VII) oxides of the type L–ReO3 are presented: [4-(trifluoromethyl)phenyl]trioxorhenium 1b, [4-(trifluoromethoxy)phenyl]trioxorhenium 2b, [4-(trifluoromethyl)tetrafluorophenyl]trioxorhenium(THF) 3b·THF and (2,2,6,6-tetramethylpiperidin-1-yl)trioxorhenium 5. As intermediate products, the novel diarylzinc compounds bis[4-(trifluoromethoxy)phenyl]zinc 2a and bis[2,6-bis(trifluoromethyl)phenyl]zinc 4a were prepared. The properties and structure of 1b–5 were studied by means of 1H, 13C, 19F and 17O NMR, IR, MS, TGA and elemental analysis. Due to the strong Lewis acidity of the Re(VII) centres crystal structures of complexes 1b and 2b were obtained as THF adducts 1b·THF and 2b·THF. Complexes 1b, 2b, 3b·THF and 5 have been examined as catalysts in olefin epoxidation using cis-cyclooctene as a model substrate. Epoxide yields of around 80% and TOFs >1300 h−1 can be obtained with 1b, 2b and 3b·THF using TBHP as an oxidant in CDCl3 at 55 °C, exceeding the only reported catalytically active aryl trioxorhenium complex xylyltrioxorhenium (XTO). Moreover, 1b shows catalytic activity in the self-metathesis of 1-hexene with good yields using Et2AlCl as a co-catalyst. Additionally, 1b and 5 were found to be efficient catalysts for the ring-opening metathesis polymerization (ROMP) of norbornene. Polynorbornene with high molecular weight can be obtained in good yields at room temperature using RnAlCl3−n as a co-catalyst. 5 is the first example of an amido trioxorhenium(VII) complex active in olefin metathesis.


 Please wait while we load your content...
Please wait while we load your content...