High temperature hydrothermal synthesis of rare-earth titanates: synthesis and structure of RE5Ti4O15(OH) (RE = La, Er), Sm3TiO5(OH)3, RE5Ti2O11(OH) (RE = Tm–Lu) and Ce2Ti4O11†
Abstract
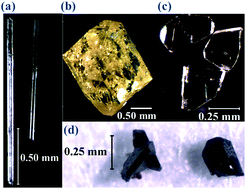
Reactions of rare-earth oxides with TiO2 were performed in high temperature (650–700 °C) hydrothermal fluids. Two different mineralizer fluids were examined, 20 M KOH and 30 M CsF, and their respective products analyzed. When concentrated KOH fluids were used, single crystals of a variety of new OH− containing species were isolated and structurally characterized: RE5Ti4O15(OH) (RE = La, Er) I, Sm3TiO5(OH)3II and RE5Ti2O11(OH) (RE = Tm–Lu) III. La5Ti4O15(OH) I crystallizes in the orthorhombic space group Pnnm with unit cell dimensions of a = 30.5152(12) Å, b = 5.5832(2) Å, c = 7.7590(3) Å and V = 1321.92(9) Å3, Z = 4. Sm3TiO5(OH)3II crystallizes in the monoclinic space group P21/m with unit cell parameters of a = 5.6066(2) Å, b = 10.4622(4) Å, c = 6.1258(2) Å and β = 104.7390(10)°, V = 347.50(2) Å3, Z = 2. Lu5Ti2O11(OH) III crystallizes in the monoclinic space group C2/m with unit cell dimensions of a = 12.1252(9) Å, b = 5.8243(4) Å, c = 7.0407(5) Å, β = 106.939(3)° and V = 475.65(6) Å3, Z = 2. When concentrated fluoride solutions are used, mostly RE2Ti2O7 type compounds were isolated in either cubic or monoclinic phases. In the case of cerium, Ce2Ti4O11IV was isolated that crystallizes in the monoclinic space group C2/c with unit cell parameters of a = 13.6875(7) Å, b = 5.0955(3) Å, c = 12.8592(7) Å, β = 108.964(2)° and V = 848.18(8) Å3, Z = 4. The synthesis, structural characterization, and supporting characterization are reported for all compounds. The work highlights the complementary nature of hydroxide and fluoride fluids in studying the reactivity of refractory oxides.


 Please wait while we load your content...
Please wait while we load your content...