Competing mechanisms and origins of chemo- and stereo-selectivities of NHC-catalyzed reactions of enals with 2-aminoacrylates†
Abstract
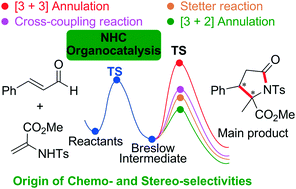
Exploring the general principle to control the various chemoselectivities of the N-heterocyclic carbene (NHC)-catalyzed reactions of enals with enamides was found to be one of the most challenging tasks in the organocatalysis field. In this paper, the origins of the chemo- and stereo-selectivities of NHC-catalyzed reactions between enals and 2-aminoacrylates have been systematically investigated by using density functional theory (DFT). After the same reaction pathway for forming the Breslow intermediate, four possible reaction pathways leading to different products, i.e., [3 + 3] annulation, cross-coupling, Stetter, and [3 + 2] annulation reaction pathways, have been studied extensively. The computational results show that the [3 + 2] annulation pathway is the most energetically favorable among the four pathways, and the (C–C) bond formation process determines the chemoselectivity and stereoselectivity of the reaction. For the chemoselectivity, the different FMO energy gaps between the reactants involved in the four reactions would be responsible for the energy favorability of the five-membered γ-lactam product. A number of non-covalent interactions (such as hydrogen bonds and C–H⋯π) between the Breslow intermediate and 2-aminoacrylate are found to contribute significantly to the stability of the most energetically favorable RS-configurational transition state. The mechanistic insights obtained in this work could provide a useful route for predicting the reactive sites of the substrates involved in this kind of reaction.


 Please wait while we load your content...
Please wait while we load your content...