Effect of the acidity of HZSM-5/MCM-41 hierarchical zeolite on its catalytic performance in supercritical catalytic cracking of n-dodecane: experiments and mechanism†
Abstract
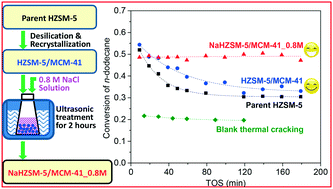
The effect of the acidity of HZSM-5/MCM-41 hierarchical zeolite on its catalytic performance in the supercritical catalytic cracking of n-dodecane was investigated by controlled ion exchange with NaCl solution under ultrasonic conditions. XRD and nitrogen adsorption–desorption results showed that the crystal and porous properties were not affected, while NH3-TPD, the probe reaction of n-hexane cracking and temperature-programmed surface reaction (TPSR) of n-dodecane demonstrated that strong Brønsted acid sites were replaced by Na+ (weak Lewis acid sites) to different extents with varying concentrations of NaCl solution. Although the initial conversion of n-dodecane during the supercritical catalytic cracking was slightly reduced, its stability was greatly enhanced by ion exchange. An ultrastable catalyst with no obvious deactivation within 3 h of time on stream (TOS) was obtained by ion exchange with 0.8 M NaCl solution. A mechanism describing the role of the acid sites residing in both micropores and mesopores of the hierarchical catalyst was proposed based on coke analysis.


 Please wait while we load your content...
Please wait while we load your content...