Efficient and stable photocatalytic NO removal on C self-doped g-C3N4: electronic structure and reaction mechanism†
Abstract
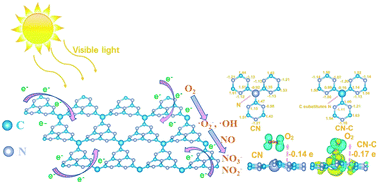
Although graphitic carbon nitride (g-C3N4, CN) has been widely studied for its photocatalytic applications in environmental remediation and solar energy conversion, the electronic structure of CN has not been optimized in terms of reactant activation and ROS generation. In this work, C self-doped g-C3N4 (CN-C) was prepared by co-pyrolysis of urea and saccharose and showed highly enhanced photocatalytic NO removal efficiency in comparison with the pristine CN. Theoretical and experimental methods were highly combined to illustrate the geometric structures of CN-C and reveal the promotion mechanisms in terms of enhanced optical and electron transfer properties. The unique electronic structure of CN-C allows NO and O2 to be more easily activated for the production of reactive radicals to participate in the photocatalytic redox reaction. The enhanced production of reactive radicals and the boosted separation rate of photogenerated carriers could promote the photocatalysis efficiency, leading to efficient and stable photocatalytic oxidation of NO over C self-doped g-C3N4. Importantly, the conversion pathways of photocatalytic NO oxidation over CN and CN-C have been elucidated based on the results of DFT calculations, ESR spectra and in situ DRIFTS spectra. A new absorption band at 2175 cm−1 associated with the NO+ intermediate is discovered for CN-C. The present work could offer new insights into the understanding of the electronic structure and photocatalytic NO oxidation mechanism on typical photocatalysts.


 Please wait while we load your content...
Please wait while we load your content...