Understanding the chemoselectivities between carbonyl and hydroxyl groups in the Rh(ii)–azavinyl carbene involved reactions†
Abstract
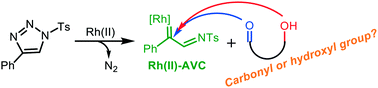
Computational studies were carried out to understand the chemoselectivities between carbonyl and hydroxyl groups in the Rh(II)–azavinyl carbene (Rh(II)–AVC) intermediate involved reactions. For the Rh(II)-catalyzed tandem reaction of 1-sulfonyl-1,2,3-triazoles with salicylaldehydes to produce 2,5-epoxy-1,4-benzoxazepines, the nucleophilic addition of the carbonyl group to the carbenoid of Rh(II)–AVC occurs more readily than the nucleophilic addition of the hydroxyl group and C–H group of the phenyl ring, affording a formal [3 + 2] cycloaddition oxazoline intermediate. A detailed mechanistic pathway for the subsequent conversion of the oxazoline intermediate to the final product was revealed. In contrast, for the Rh-catalyzed reactions of 1-sulfonyl-1,2,3-triazoles with 4-hydroxyacetophenone and with α,β-unsaturated cyclic ketones bearing an aliphatic hydroxyl group, the nucleophilic addition of the hydroxyl group to the carbenoid of Rh(II)–AVC is more favorable than the nucleophilic addition of the carbonyl group. The main factors responsible for the preferred nucleophilic addition of carbonyl/hydroxyl groups to the carbenoid of Rh(II)–AVC were discussed.


 Please wait while we load your content...
Please wait while we load your content...