On the molecular optical nonlinearity of halogen-bond-forming azobenzenes
Abstract
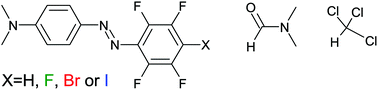
We study hyper-Rayleigh scattering and computed molecular hyperpolarizability in a series of azobenzene chromophores in chloroform and dimethylformamide as solvents. The chromophores form halogen or hydrogen bonds of varying strength with dimethylformamide molecules, differently from what is expected for chloroform. We show that hyperpolarizability is unaffected or sligthly lower with the azobenzene forming the strongest halogen bond. Solid supramolecular polymers with the same chromophores have previously demonstrated clearly higher second-order nonlinear responses when a halogen-bond-accepting polymer is used, the larger increase being associated with the stronger halogen bond. The present study proves that the higher optical nonlinearity in polymers lies in the better ordering of the chromophores instead of changes in molecular hyperpolarizability, highlighting the unique properties of halogen bonding in supramolecular chemistry.


 Please wait while we load your content...
Please wait while we load your content...