Identification and computational characterization of isomers with cis and trans amide bonds in folate and its analogues†
Abstract
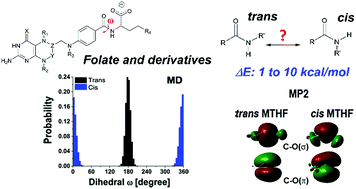
Folate and its synthetic analogues, called antifolates, are known to have diverse bio-applications, for example as cell proliferation stimulators or anticancer drugs. Their molecular structure is important for performing the required biological activity. Since all folate-derived ligands contain a peptide-like amide bond, its configuration is one of the key components for the functional fitness of such compounds. During the modelling of folate and three of its derivatives – methotrexate, 5-methyl tetrahydrofolate, and pteroyl ornithine, we registered significant population of the cis isomers along the amide bond. The properties of the cis and trans forms of the ligands in saline are studied in detail by classical atomistic molecular dynamics and by quantum chemical methods. The calculations predict high probability for coexistence of the cis isomers for two of the ligands. The energetic instability of the cis form is explained with a σ-character admixture into the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O(π) bond, while its magnitude is attributed to the pattern of local electron density redistribution. The cis forms of all molecules have markedly slower structural dynamics than the trans ones, which might affect their behavior in vivo.
O(π) bond, while its magnitude is attributed to the pattern of local electron density redistribution. The cis forms of all molecules have markedly slower structural dynamics than the trans ones, which might affect their behavior in vivo.


 Please wait while we load your content...
Please wait while we load your content...