Comment on “Investigations on HONO formation from photolysis of adsorbed HNO3 on quartz glass surfaces” by S. Laufs and J. Kleffmann, Phys. Chem. Chem. Phys., 2016, 18, 9616
Abstract
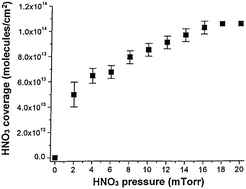
Laufs and Kleffmann observed that HNO3 surface photolysis rates resemble that of HNO3 in the gas phase after depositing HNO3 in air at ∼50% relative humidity onto quartz glass surfaces. They questioned the dry HNO3 coverage (1.1 × 1014 molecules per cm2 after depositing ∼15 mTorr HNO3 on silica at 0% humidity) used to derive our previously published HNO3 near-UV surface absorption cross sections. We directly determined the HNO3 coverage on a quartz surface using a quartz crystal microbalance (QCM). A similar HNO3 monolayer coverage obtained by QCM confirms that our estimated HNO3 coverage is reasonable. We also obtained an NO2 quantum yield from the 308 nm HNO3 photolysis on fused silica. In this Comment, we provide an explanation of the variance in HNO3 surface photolysis rates by clarifying the effects arising from important differences in the HNO3 coverage on quartz/silica in the presence of humidity versus those in the absence of humidity.


 Please wait while we load your content...
Please wait while we load your content...