Spectroscopic properties of LaGaO3:V,Nd3+ nanocrystals as a potential luminescent thermometer†
Abstract
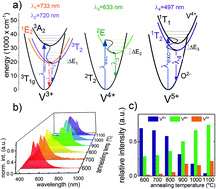
In this work we present the spectroscopic properties of LaGaO3:V,Nd3+ nanocrystals, which have been successfully obtained by the Pechini method. This is the first study where vanadium ions were applied in a LaGaO3 lattice for a non-contact luminescent thermometer. It was found that vanadium ions in the LaGaO3 matrix appear in three oxidation states, namely V5+, V4+ and V3+. It was found that the relative emission intensities of various states of vanadium ions depend strongly on grain size and therefore the emission color of LaGaO3:V can be easily modulated via the annealing temperature. The spectroscopic properties of this material were investigated in a wide temperature range (−150–300 °C). It was found that in the case of V-singly doped nanocrystals, the V4+ ions, reveal the best temperature sensing performance with high relative sensitivity (S = 1.76% °C−1) and broad usable temperature range (−50–150 °C). The different rates of thermal luminescence quenching of the vanadium ions provide three forms of non-contact temperature sensor, namely LaGaO3:V5+,Nd3+, LaGaO3:V4+,Nd3+ and LaGaO3:V3+,Nd3+. The highest sensitivities were found to be 1% °C−1 (at −5 °C and 90 °C), 0.49% °C−1 (at −20 °C) and 1.44% °C−1 (at 75 °C) for LaGaO3:V5+,Nd3+, LaGaO3:V4+,Nd3+ and LaGaO3:V3+,Nd3+, respectively.


 Please wait while we load your content...
Please wait while we load your content...