Thermodynamic and kinetic isotope effects on the order–disorder transition of ice XIV to ice XII
Abstract
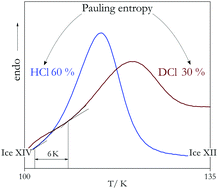
Isotope effects accompanying the order–disorder transition of ice XIV to ice XII are studied using calorimetry, X-ray diffraction, and dielectric spectroscopy. Particular emphasis is placed on the impact of the cooling rate applied during high-pressure production and during ambient-pressure recooling on the degree of hydrogen order in the low-temperature ice XIV phase. For specimens from D2O, ordering is harder to achieve in the sense that despite smaller cooling rates, the degree of order is less than in crystals produced from H2O. The degree of ordering can be quantified in terms of the Pauling entropy using calorimetry and manifests itself in structural and dynamical features that were examined using X-ray diffraction and dielectric spectroscopy, respectively. In hydrogen chloride doped samples, H/D substitution was found to slow down the dipolar dynamics up to about 30-fold and shifts the order–disorder transition by 4–6 K. By contrast to earlier assumptions it is possible to reach a high degree of ordering also at ambient pressure, provided the cooling rate is small enough. That is, at ambient pressure, orthorhombic stress slows down the dipolar reorientation near the ordering transition by a factor of 300–2000 for H2O and 30–100 for D2O samples. Furthermore, by long-term storage of our samples at 77 K we have reached surprisingly large increases in degree of order. For the D2O samples we observed an unprecedented high order, corresponding to more than 45% of the Pauling entropy.


 Please wait while we load your content...
Please wait while we load your content...