Nature of halogen bonding involving π-systems, nitroxide radicals and carbenes: a highlight of the importance of charge transfer†
Abstract
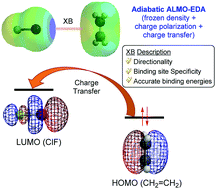
The recently developed adiabatic absolutely localized molecular orbital energy decomposition analysis (ALMO-EDA) has proven to be useful in determining the effects of different energy components on the geometries of complexes bound by intermolecular interactions. The authors have applied it to systems such as the water dimer, water–ion complexes, metallocenes and lone-pair type halogen-bonded (XB) dimers. In this study, we have extended the second-generation ALMO-EDA method to 40 different XB complexes by benchmarking against its classical counterpart and symmetry-adapted perturbation theory (SAPT). In addition, we have examined the nature of halogen bonding involving less studied XB acceptors, namely π-systems, radicals and carbenes, using the adiabatic ALMO-EDA analyses, particularly to shed light on how each energy component affects the geometries of the XB complexes. Our results show that the second-generation ALMO-EDA predicts a higher electrostatic energy contribution in all XB complexes compared to SAPT and classical ALMO-EDA schemes. On the other hand, when comparing across different XB acceptors, all three partition schemes produced the same qualitative finding. The adiabatic ALMO-EDA analyses indicate that while the inclusion of a charge transfer contribution is important in achieving accurate XB bond lengths and interaction energies, as well as recovering the binding site specificity of XB involving benzene and naphthalene acceptors, it is sufficient to obtain the linearity of the XB complexes in the frozen approximation.


 Please wait while we load your content...
Please wait while we load your content...