Temperature-dependent crystalline structure and phase transition of poly(butylene adipate) end-functionalized by multiple hydrogen-bonding groups
Abstract
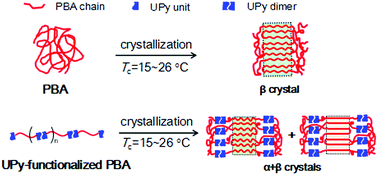
Because of the different crystallization behaviors of semicrystalline supramolecular polymers (SMPs) from conventional polymers, investigation on the unique crystallization kinetics and crystalline structures of SMPs is of fundamental importance to tune their physical properties and functions in processing. Herein, we chose the multiple hydrogen-bonding 2-ureido-4[1H]-pyrimidinone (UPy) group as the supramolecular unit and poly(butylene adipate) (PBA) as the polymorphic polymer block, and synthesized UPy-functionalized PBAs (i.e., UPy-bonded supramolecular PBAs). The crystallization kinetics, polymorphic crystalline structure, phase transition, and lamellar morphology of the UPy-functionalized PBAs were investigated and compared with those of the pristine PBA. UPy end functionalization suppressed the crystallization rate and crystallinity of the linked PBA chains. Compared to the pristine PBA, the UPy-functionalized PBAs preferred to form the thermally-stable α crystals at the same temperature; this was more obvious for the samples with a high content of the UPy end group. The facilitated formation of α crystals in the UPy-functionalized PBAs was attributed to the decreased equilibrium melting temperature. UPy end functionalization also decreased the critical temperature and broadened the temperature range for the β-to-α phase transition of PBA during heating. Due to the segregation of the UPy unit in the amorphous phase, the UPy-functionalized PBAs exhibited larger long periods than the pristine PBA, even though they had a lower degree of crystallinity.


 Please wait while we load your content...
Please wait while we load your content...