DFT studies on quantum mechanical tunneling in tautomerization of three-membered rings†
Abstract
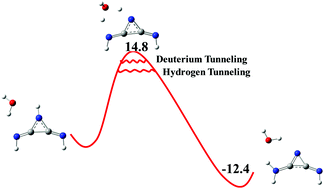
We have examined keto–enol and amino–imino tautomerization in a set of three-membered ring systems (1–5) in the absence and presence of water molecules. Aromaticity governs the keto–enol and amino–imino tautomerization processes in (1–5), which lead to the formation of enol and imine derivatives. The possibility of quantum mechanical tunneling (QMT) has not been reported in the tautomerization processes of three-membered ring systems. M062X/6-311+G(d,p) level of theory QMT calculations reveal that tunneling is not possible in the water unassisted processes because of very high free energy activation barriers. The activation free energy barriers for the amino–imino tautomerization of 5, aziridine-2,3-diimine, and one water assisted, 5-W, are 58.1 kcal mol−1 and 14.8 kcal mol−1, respectively and the lowest among the 3-membered rings examined. The classical over the barrier rate constant (kCVT) obtained by QMT calculation for 5-W→5-W-P is 10.6 s−1 at 273 K. Inclusion of small curvature tunneling (SCT) enhances the classical over the barrier rate constant by 15.1 times at 273 K, i.e., kCVT+SCT is 160 s−1 and reveals nonclassical behaviour for the tautomerization of 5-W. A higher kinetic isotope effect in the tautomerization process of 4-W and 5-W also indicates a pronounced contribution of tunneling toward the tautomerization process. The two-water assisted tautomerization of 3 has the highest activation free energy barrier in the series indicating a nonclassical contribution to 3-2W→3-2W-P. These results suggest that the tautomerization processes of 1–5 are experimentally feasible by tunneling and aromaticity.


 Please wait while we load your content...
Please wait while we load your content...