Detailed kinetics of tetrafluoroethene ozonolysis†
Abstract
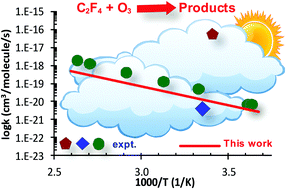
The C2F4 + O3 reaction plays an important role in the oxidation process of perfluoroalkenes in the atmosphere. The detailed reaction mechanism was explored using the accurate electronic structure method, CCSD(T)/CBS//B3LYP/aug-cc-pVTZ. The 1,3-cycloaddition of O3 with C2F4 to form the primary ozonide was found to be the rate-determining step of the oxidation process with a small barrier (i.e., 7.3 kcal mol−1 at 0 K). The temperature- and pressure-dependent behaviors of the title reaction were characterized in the range of 200–1000 K & 0.1–760 Torr using the integrated deterministic and stochastic master equation/Rice–Ramsperger–Kassel–Marcus (ME/RRKM) rate model with the inclusion of the corrections for anharmonicity and tunneling treatments. It is found that the anharmonic effect plays a role in the kinetic behaviors (e.g., lower the rate by a factor of ∼ two at 298 K) while the tunneling correction is insignificant. The total rate constants were found to be pressure-independent under the considered conditions, shown as ktot(T) = 4.80 × 10−23 × T2.69 × exp(−2983.4 K/T) (cm3 per molecule per s), which confirms the latest experimental data by Acerboni et al. (G. Acerboni, N. R. Jensen, B. Rindone and J. Hjorth, Chem. Phys. Lett., 1999, 309, 364–368); thus this study helps to resolve a long-term controversy among the previous measurements. The sensitivity analyses on the derived rate coefficients and time-resolved species mole fraction with respect to the ab initio input parameters were also performed to further understand as well as quantify the kinetic behaviors for the title reaction.


 Please wait while we load your content...
Please wait while we load your content...