Improving the activity of gold nanoparticles for the water-gas shift reaction using TiO2–Y2O3: an example of catalyst design
Abstract
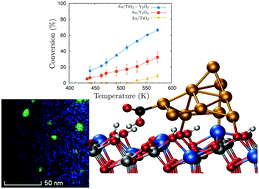
In the last ten years, there has been an acceleration in the pace at which new catalysts for the water-gas shift reaction are designed and synthesized. Pt-based catalysts remain the best solution when only activity is considered. However, cost, operation temperature, and deactivation phenomena are important variables when these catalysts are scaled in industry. Here, a new catalyst, Au/TiO2–Y2O3, is presented as an alternative to the less selective Pt/oxide systems. Experimental and theoretical techniques are combined to design, synthesize, characterize and analyze the performance of this system. The mixed oxide demonstrates a synergistic effect, improving the activity of the catalyst not only at large-to-medium temperatures but also at low temperatures. This effect is related to the homogeneous dispersion of the vacancies that act both as nucleation centers for smaller and more active gold nanoparticles and as dissociation sites for water molecules. The calculated reaction path points to carboxyl formation as the rate-limiting step with an activation energy of 6.9 kcal mol−1, which is in quantitative agreement with experimental measurements and, to the best of our knowledge, it is the lowest activation energy reported for the water-gas shift reaction. This discovery demonstrates the importance of combining experimental and theoretical techniques to model and understand catalytic processes and opens the door to new improvements to reduce the operating temperature and the deactivation of the catalyst.


 Please wait while we load your content...
Please wait while we load your content...