Pentavalent phosphorus as a unique phosphorus donor in POCl3 homodimer and POCl3–H2O heterodimer: matrix isolation infrared spectroscopic and computational studies†
Abstract
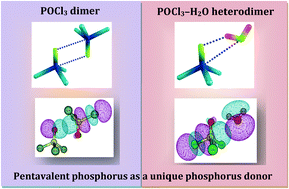
Phosphorus, an important element among the pnicogen group, opens up avenues for experimental and computational explorations of its interaction in a variety of compounds. Although experimental proof of trivalent phosphorus bonding is limited and is growing with time, phosphorus bonding with pentavalent phosphorus has been a long sought after interaction both computationally and experimentally. In the present work, for the first time, we have provided unambiguous experimental evidence for the pentavalent phosphorus bonding interaction by exploiting a phosphoryl chloride (POCl3) prototype under isolated conditions at low temperatures. The POCl3 dimer and higher aggregates can be set as a unique example possessing pentavalent phosphorus bonding with a competing halogen bonding interaction. The POCl3–H2O heterodimer is another interesting system, stabilized through multiple phosphorus and hydrogen bonded interactions. Using matrix isolation infrared spectroscopy, the POCl3 homodimer and POCl3–H2O heterodimer were characterized and the structures were elucidated by employing ab initio and DFT methods. The multifaceted interactions in the POCl3 paradigm were investigated by using Natural Bond Orbital, Energy Decomposition and Electrostatic Potential Mapping analyses.


 Please wait while we load your content...
Please wait while we load your content...