The local structure in the BmimPF6/acetonitrile mixture: the charge distribution effect
Abstract
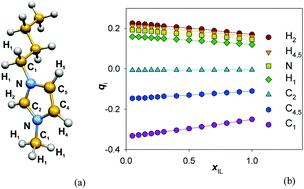
The changes of the local structure in the binary mixture of 1-butyl-3-methylimidazolium hexafluorophosphate (BmimPF6) ionic liquid and acetonitrile are investigated over the entire composition range. Two charge distribution models of the ions are considered: in the first one, the atomic fractional charges of the cations and anions are kept equal with those in the neat ionic liquid, and hence they are independent from the mole fraction of the ionic liquid, while in the second one the charge distribution is scaled up by a mole fraction dependent factor. The sum of these charges converge to +1e and −1e on the cation and anion, respectively, at infinite dilution. All the other interactions and geometry parameters of the ions (i.e., Lennard-Jones, bond stretching, angle bending and dihedral parameters) are identical in the two cases. The effect of the fractional charge distribution on the hydrogen bonding between the counterions themselves and between the ions and solvent molecules, as well as on the stacking interactions between the cations, is analyzed. To this end, two distances, characteristic of the hydrogen bond formed by the donor moiety and its nearest neighbor acceptor, as well as a coordinate system that defines unambiguously the orientation between a reference cation and its nearest neighbor, are introduced. It is shown that, with the variable charge model, the neighboring cation–anion pairs maintain their relative arrangement similar to the neat ionic liquid down to an ionic liquid mole fraction of xIL = 0.10, whereas in the case of the constant charge model such changes occur already at xIL = 0.20. Furthermore, the analysis of the first and the second nearest neighbor distance distributions of an anion around a reference cation indicates that, at this mole fraction range, there are two different preferred arrangements of the anions around the cations. In the first one, similarly to the local structure around a reference cation in the neat ionic liquid, the anion forms a distorted hydrogen bond with the cation, while in the second one the anion is located farther from the cation, forming no hydrogen bond with it. The relative population of these two types of preferred nearest neighbor cation–anion arrangements is found to be sensitive to further decrease of the ionic liquid mole fraction. These findings correlate with experimental results concerning the behavior of many physical chemical properties (e.g., excess volume, excess viscosity, chemical shift, infrared and Raman vibrational mode shifts, diffusion, etc.) that were found to undergo a drastic change in this mole fraction range. Our results show that in this composition range a transition occurs from the situation where the macroscopic physical chemical properties of the system are determined primarily by the cation–anion hydrogen bonding interactions to that where they are determined by the solvation of the cation and the anion by the molecular solvent.
- This article is part of the themed collection: 2018 PCCP HOT Articles


 Please wait while we load your content...
Please wait while we load your content...