The bismuth tetramer Bi4: the ν3 key to experimental observation†
Abstract
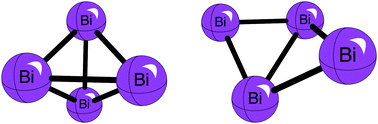
The spectroscopic identification of Bi4 has been very elusive. Two constitutional Bi4 isomers of Td and C2v symmetry are investigated and each is found to be a local energetic minimum. The optimized geometries and vibrational frequencies of these two isomers are obtained at the CCSD(T)/cc-pVQZ-PP level of theory, utilizing the Stoll, Metz, and Dolg 60-electron effective core potential. The fundamental frequencies of the Td isomer are obtained at the same level of theory. The focal point analysis method, from a maximum basis set of cc-pV5Z-PP, and proceeding to a maximum correlation method of CCSDTQ, was employed to determine the dissociation energy of Bi4 (Td) into two Bi2 and the adiabatic energy difference between the C2v and Td isomers of Bi4. These quantities are predicted to be +65 kcal mol−1 and +39 kcal mol−1, respectively. Two electron vertical excitation energies between the Td and C2v electronic configurations are computed to be 156 kcal mol−1 for the Td isomer and 9 kcal mol−1 for the C2v isomer. The most probable approach to laboratory spectroscopic identification of Bi4 is via an infrared spectrum. The predicted fundamentals (cm−1) with harmonic IR intensities in parentheses (km mol−1) are 94(0), 123(0.23), and 167(0) for the Td isomer. The moderate IR intensity for the only allowed fundamental may explain why Bi4 has yet to be observed. Through natural bond orbital analysis, the C2v isomer of Bi4 was discovered to exhibit “long-bonding” between the furthest apart ‘wing’ atoms. This long-bonding is postulated to be facilitated by the σ-bonding orbital between the ‘spine’ atoms of the C2v isomer.
- This article is part of the themed collection: 2018 PCCP HOT Articles


 Please wait while we load your content...
Please wait while we load your content...