Mechanism of the thermal decomposition of tetramethylsilane: a flash pyrolysis vacuum ultraviolet photoionization time-of-flight mass spectrometry and density functional theory study†
Abstract
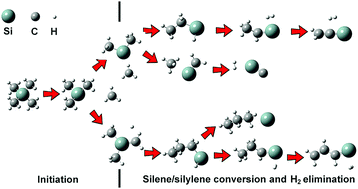
The thermal decomposition of tetramethylsilane (TMS) was studied over the temperature range of 298–1450 K by combining flash pyrolysis vacuum ultraviolet photoionization time-of-flight mass spectrometry (VUV-PI-TOFMS) and density functional theory (DFT). The initial step in TMS pyrolysis produced a methyl radical (Me˙) and Me3Si˙. Me3Si˙ underwent subsequent loss of a hydrogen atom to form Me2Si![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH2 and loss of a methyl radical to form Me2Si:. Isomerizations via 1,2-shift and H2 eliminations were major secondary decomposition reactions of Me2Si
CH2 and loss of a methyl radical to form Me2Si:. Isomerizations via 1,2-shift and H2 eliminations were major secondary decomposition reactions of Me2Si![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH2 and Me2Si:. Among the various isomers, silylene species containing Si–H bonds, such as :Si(H)CH2CH2CH3, :Si(H)CH2CH
CH2 and Me2Si:. Among the various isomers, silylene species containing Si–H bonds, such as :Si(H)CH2CH2CH3, :Si(H)CH2CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH2, :Si(H)CH2CH3, and :Si(H)CH
CH2, :Si(H)CH2CH3, and :Si(H)CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH2, played an important role in H2 elimination reactions. On the other hand, silene species were insignificant in H2 eliminations. Unlike the silylene species, H2 elimination of :Si
CH2, played an important role in H2 elimination reactions. On the other hand, silene species were insignificant in H2 eliminations. Unlike the silylene species, H2 elimination of :Si![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH2 was energetically unfavorable.
CH2 was energetically unfavorable.


 Please wait while we load your content...
Please wait while we load your content...