A molecular dynamics investigation of actinyl–ligand speciation in aqueous solution†
Abstract
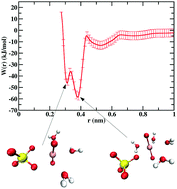
Actinyl ions (AnO2n+), the form in which actinides are commonly found in aqueous solution, are important species in the nuclear fuel cycle. These ions can form stable complexes with ionic ligands such as OH−, NO3−, Cl−, and even other actinyl ions in the aqueous phase. Knowledge of the relative stabilities of these complexes is important for the efficient design of separation processes used in recycling. These complexes also play a major role in the formation of actinide nanoclusters. A quantitative treatment of the stability of these actinyl ion complexes is therefore warranted. In the present work, molecular dynamics (MD) simulations have been performed to calculate the potential of mean force (PMF) between two actinyl ions (UO22+ and NpO2+) and various ligands (F−, Cl−, OH−, NO3−, SO42−, CO32−, Na+, and H2O) in explicitly-modeled aqueous solution. Equilibrium constants were calculated from the PMFs, and are consistent with experimental trends. Dication actinyls show stronger affinity for anions compared to monocation actinyls, whereas the opposite is true for cation–cation interactions between actinyls. Finally, the dynamics of actinyl–ligand contact ion pair (CIP) dissociation were characterized by calculating rate constants from transition state theory. The transmission coefficient, a dynamical correction factor used to correct for reaction barrier recrossing, was calculated for each actinyl–ligand CIP dissociation event.
- This article is part of the themed collection: 2018 PCCP HOT Articles


 Please wait while we load your content...
Please wait while we load your content...