Diabatic potential energy surfaces of MgH2+ and dynamic studies for the Mg+(3p) + H2 → MgH+ + H reaction
Abstract
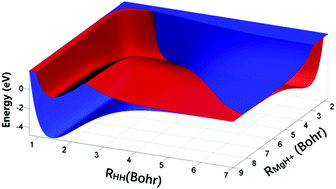
The global diabatic potential energy surfaces (PESs) of the MgH2+ system, which can be used to study the dynamics of the Mg+(3p2P) + H2(X1Σ+g) → MgH+(X1Σ+) + H(2S) reaction, are structured for the first time. The diabatic PESs are correlated with the ground state 12A′ and the first excited state 22A′. The multi-reference configuration interaction method and the VQZ basis set are used in ab initio calculations, and diabatic potential energies are calculated based on the molecular properties of the dipole moment. The neural network method is applied to fit the matrix elements of the diabatic energy surfaces. Spectroscopic constants of H2(X1Σg+) and MgH+(X1Σ+) obtained from the new PESs agree well with the experimental data. Based on the diabatic PESs of MgH2+, the time-dependent wavepacket calculations for the Mg+(3p2P) + H2(X1Σg+) → MgH+(X1Σ+) + H(2S) reaction are carried out to study the reaction dynamics. There is no threshold for this reaction because of the existence of barrierless reactive paths. The reaction has a high total integral cross section (ICS), and vibrationally resolved ICSs show an obvious population inversion of product vibrational states. The results of differential cross sections (DCSs) indicate that most product molecules tend to forward scatter.


 Please wait while we load your content...
Please wait while we load your content...