Regioselective copper-catalyzed aminoborylation of styrenes with bis(pinacolato)diboron and diazo compounds†
Abstract
A Cu(I)-catalyzed aminoborylation reaction of styrenes is reported. In this transformation, diazo compounds are used as the electrophilic amination agent. The in situ generated benzylcopper species is trapped by the electrophilic nitrogen terminus of the diazo substrate to afford borylated hydrazones in a regioselective manner.
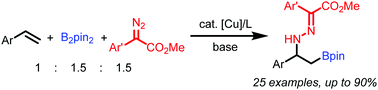


 Please wait while we load your content...
Please wait while we load your content...