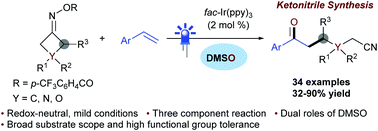
A photoredox catalyzed iminyl radical-triggered C–C bond cleavage/addition/Kornblum oxidation cascade of oxime esters and styrenes: synthesis of ketonitriles†
Abstract
A photoredox-catalyzed iminyl radical-triggered C–C bond cleavage/addition/Kornblum oxidation cascade of cycloketone oxime esters and styrenes in DMSO is described. This three-component, one-pot procedure features mild conditions, a broad substrate scope, and high functional group tolerance, providing an efficient approach to access diversely functionalized ketonitriles.


 Please wait while we load your content...
Please wait while we load your content...