A facile route to 1H- and 2H-indazoles from readily accessible acyl hydrazides by exploiting a novel aryne-based molecular rearrangement†
Abstract
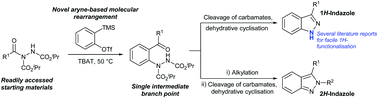
Herein we report the transformation of readily synthesised acyl hydrazides into 2-hydrazobenzophenones via a novel molecular rearrangement pathway using aryne chemistry. The developed reaction protocol is performed under relatively mild conditions and is tolerant of a wide variety of functional groups, and the 2-hydrazobenzophenone products provide access to both 1H- and 2H-indazoles from a single intermediate.


 Please wait while we load your content...
Please wait while we load your content...