Cu transfer from amyloid-β4–16 to metallothionein-3: the role of the neurotransmitter glutamate and metallothionein-3 Zn(ii)-load states†
Abstract
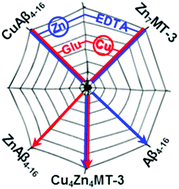
Copper transfer from Cu(II)amyloid-β4–16 to human Zn7-metallothionein-3 can be accelerated by glutamate and by lowering the Zn-load of metallothionein-3 with EDTA. Glutamate facilitates the Cu(II) release, and Zn4–6-metallothionein-3 react more rapidly. These mechanisms are additive, proving the intricate and interconnected network of zinc and copper trafficking between biomolecules.


 Please wait while we load your content...
Please wait while we load your content...