Monitoring alkaline transitions of yeast iso-1 cytochrome c at natural isotopic abundance using trimethyllysine as a native NMR probe†
Abstract
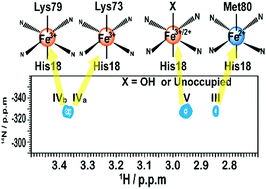
Spectral overlap makes it difficult to use NMR for mapping the conformational profile of heterogeneous conformational ensembles of macromolecules. Here, we apply a 1H–14N HSQC experiment to monitor the alkaline conformational transitions of yeast iso-1 cytochrome c (ycyt c) at natural isotopic abundance. Trimethylated Lys72 of ycyt c is selectively detected by a 1H–14N HSQC experiment, and used as a probe to trace conformational transitions of ycyt c under alkaline conditions. It was found that at least four different conformers of ycyt c coexisted under alkaline conditions. Besides the native structure, Lys73 or Lys79 coordinated conformers and a partially unfolded state with exposed heme were observed. These results indicate that the method is powerful at simplifying spectra of a trimethylated protein, which makes it possible to study complex conformational transitions of naturally extracted or chemically modified trimethylated protein at natural isotopic abundance.


 Please wait while we load your content...
Please wait while we load your content...