Synthesis of multi-substituted dihydrofurans via palladium-catalysed coupling between 2,3-alkadienols and pronucleophiles†
Abstract
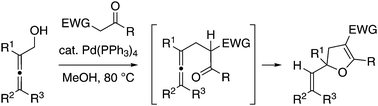
Multi-substituted dihydrofurans were obtained from a palladium-catalysed coupling reaction between 2,3-alkadienols and ketones bearing an electron-withdrawing group at the α-position. Methanol as a solvent was essential for the initial dehydrative substitution to suppress the competitive hydroalkylation of the diene moiety. The substitution would be followed by intramolecular hydroalkoxylation under the same catalysis.


 Please wait while we load your content...
Please wait while we load your content...