Highly hindered 2-(aryl-di-tert-butylsilyl)-N-methyl-imidazoles: a new tool for the aqueous 19F- and 18F-fluorination of biomolecule-based structures†
Abstract
A new class of silicon-based fluoride acceptors with a C-linked heterocycle as the leaving group was synthesized in one step from commercial chemicals, and linked to biomolecules. The resulting conjugates were efficiently 19F-fluorinated in aqueous mixtures, and switching to 18F-labelling provided nucleoside- and peptide-based bioconjugates with excellent molar activities suitable for biological applications.
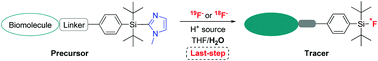


 Please wait while we load your content...
Please wait while we load your content...