1,3-Iodo-amination of 2-methyl indoles via Csp2–Csp3 dual functionalization with iodine reagent†
Abstract
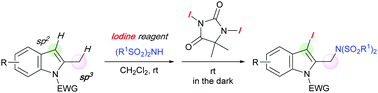
A 1,3-iodo-amination with iodine reagent that involved the Csp2–Csp3 dual functionalization of 2-methyl indoles was developed to provide 2-aminomethyl-3-iodo-indole derivatives. The iodo-amination proceeded via a 1,4-transfer of an imide group through the formation of an indolyl(phenyl)iodonium imide using PhI(OAc)2, followed by an iodination using DIH or a double iodination of indole using excess DIH.


 Please wait while we load your content...
Please wait while we load your content...