Catalytic asymmetric formal total syntheses of (+)- and (−)-cycloclavine†
Abstract
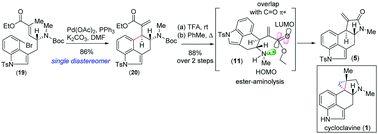
We report an expeditious catalytic asymmetric approach to clavine alkaloids via a key Heck cyclization. This reaction sets the formation of vicinal stereocenters with excellent diastereoselectivity. Utilizing the aforementioned strategy, the formal total synthesis of cycloclavine (1) has been achieved via another key late-stage ester-aminolysis of 6.
- This article is part of the themed collection: Natural product synthesis


 Please wait while we load your content...
Please wait while we load your content...