Epimerization-free access to C-terminal cysteine peptide acids, carboxamides, secondary amides, and esters via complimentary strategies†
Abstract
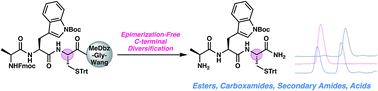
C-Terminal cysteine peptide acids are difficult to access without epimerization of the cysteine α-stereocenter. Diversification of the C-terminus after solid-phase peptide synthesis poses an even greater challenge because of the proclivity of the cysteine α-stereocenter to undergo deprotonation upon activation of the C-terminal carboxylic acid. We present herein two general strategies to access C-terminal cysteine peptide derivatives without detectable epimerization, diketopiperazine formation, or piperidinylalanine side products.
- This article is part of the themed collection: Collection to celebrate our diverse and global authorship


 Please wait while we load your content...
Please wait while we load your content...