Selective electrochemical generation of benzylic radicals enabled by ferrocene-based electron-transfer mediators†
Abstract
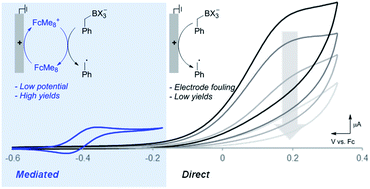
The generation and intermolecular functionalisation of carbon-centred radicals has broad potential synthetic utility. Herein, we show that benzylic radicals may be generated electrochemically from benzylboronate derivatives at low electrode potentials (ca. −0.3 V vs. Cp2Fe0/+) via single electron oxidation. Use of a catalytic quantity of a ferrocene-based electron-transfer mediator is crucial to achieve successful radical functionalisation and avoid undesirable side reactions arising from direct electrochemical oxidation or from the use of stoichiometric ferrocenium-based oxidants.
- This article is part of the themed collection: Collection to celebrate our diverse and global authorship


 Please wait while we load your content...
Please wait while we load your content...