Trihalide ionic liquids as non-volatile oxidizing solvents for metals†
Abstract
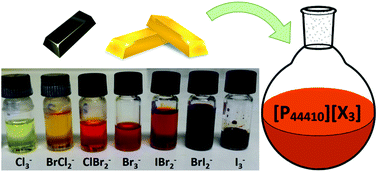
Various ionic liquids containing a trihalide anion [X3]− (X = Cl, Br or I) were synthesized by addition of molecular chlorine, bromine or iodine (X2) to a halide ionic liquid precursor, forming the trihalide anion via a Lewis acid–base reaction. All ionic liquids were characterized by 1H NMR, 13C NMR, 31P NMR, and Raman spectroscopy. Phosphonium trihalide ionic liquids [PR4][X3] (X = Cl, Br or I) were selected because of their low viscosity, low melting point and stability towards chlorine. The melting point, viscosity and density were measured for all ionic liquids. A series of seven trihalide ionic liquids (with anions ranging from trichloride to triiodide), containing both single trihalide and mixed trihalide anions, was prepared for the tributyldecylphosphonium cation. The effect of the trihalide anion on the physical properties of the phosphonium ionic liquids was determined using this series of ionic liquids. Trihalide anions, and polyhalides in general, are strongly oxidizing agents due to their charge deficit with respect to the number of halogen atoms. This strong oxidizing power has been applied to oxidatively dissolve several metals into tributyldecylphosphonium tribromide [P44410][Br3]. A broad range of metals was tested: Fe, Cu, Sb, Co, Zn, In, Ga, Bi, Ge, Sn, Pd, Au, Rh and Pt. With the exception of Pt and Rh, all the metals dissolved in the ionic liquid. The dissolution of Cu in six different trihalide ionic liquids was performed to confirm that these trihalide ionic liquids have similar oxidizing properties to [P44410][Br3]. Due to the negligible vapor pressure of ionic liquids, the entire oxidative dissolution process proceeded in a non-volatile solvent and without the formation of gases. This work can contribute to the development of safe, solvometallurgical methods for oxidative dissolution of metals.
- This article is part of the themed collection: 2018 Green Chemistry Hot Articles


 Please wait while we load your content...
Please wait while we load your content...