Stable Fe(iii) phenoxyimines as selective and robust CO2/epoxide coupling catalysts†
Abstract
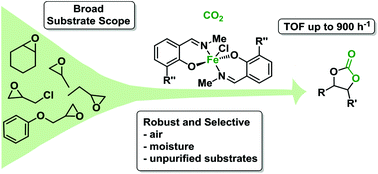
Three phenoxyimine Fe(III)Cl complexes bearing electronically diverse -Cl, -H or -tBu substituents in the ortho position were synthesised. X-ray crystallographic analysis of the complexes reveals mononuclear structures with pentacoordinate iron centres and trigonal bipyramidal geometries. All three complexes demonstrated excellent catalytic activities towards CO2/epoxide coupling to selectively form cyclic carbonates, with catalyst activity correlating with the electron withdrawing nature of the ortho-substituent (Cl > H > tBu) and thus the Lewis acidity of the metal centre. The chloro-substituted complex displayed remarkable activity in the synthesis of propylene carbonate from propylene oxide and CO2, reaching turnover frequencies (TOF) up to 760 h−1 in the presence of TBABr co-catalyst at 120 °C and 20 bars of CO2 pressure. Importantly, the catalyst is also very robust, functioning with high substrate loading, under air or in the presence of water. The substrate scope was successfully extended to other terminal epoxides including epichlorohydrin (TOF = 900 h−1) and to the more challenging internal epoxide, cyclohexene oxide (TOF = 80 h−1). These are amongst the highest TOF values reported for an iron CO2/epoxide coupling catalyst to date.
- This article is part of the themed collection: Celebrating our 2021 Prizewinners


 Please wait while we load your content...
Please wait while we load your content...