Defect generation in TiO2 nanotube anodes via heat treatment in various atmospheres for lithium-ion batteries†
Abstract
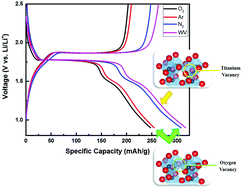
In this paper, ordered TiO2 nanotubes were grown on a Ti substrate via electrochemical anodization and subsequently annealed at 450 °C for 4 h under various atmospheres to create different point defects. Oxygen-deficient environments such as Ar and N2 were used to develop oxygen vacancies, while a water vapor (WV) atmosphere was used to generate titanium vacancies. Computational models by density functional theory predicted that the presence of oxygen vacancies would cause electronic conductivity to increase, while the presence of Ti vacancies could lead to decreased conductivity. The predictions were confirmed by two-point electrical conductivity measurements and Mott–Schottky analysis. Raman spectroscopy was also conducted to confirm the presence of defects. The annealed samples were then evaluated as anodes in lithium-ion batteries. The oxygen-deficient samples had an improvement in capacity by 10% and 25% for Ar- and N2-treated samples, respectively, while the WV-treated sample displayed a capacity increase of 24% compared to the stoichiometric control sample (annealed in O2). Electrochemical impedance spectroscopy studies revealed that the WV-treated sample's increased capacity was a consequence of its higher Li diffusivity. The results suggest that balanced electrical and ionic conductivity in nanostructured metal oxide anodes can be tuned through defect generation using heat treatments in various atmospheres for improved electrochemical properties.
- This article is part of the themed collection: 2018 PCCP HOT Articles


 Please wait while we load your content...
Please wait while we load your content...