Solvation of 2-(hydroxymethyl)-2,5,7,8-tetramethyl-chroman-6-ol revealed by circular dichroism: a case of chromane helicity rule breaking†
Abstract
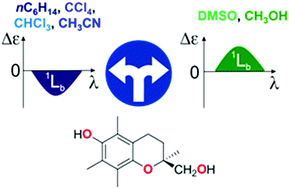
The primary goal of this work is to clarify why 2-(hydroxymethyl)-2,5,7,8-tetramethyl-chroman-6-ol {(S)-TMChM} deviates from the chromane helicity rule under solvent change. The rule, applicable to determining the absolute configuration of molecules containing the chromane chromophore, binds the sign of the 1Lb Cotton effect (CE) with the helicity of the dihydropyran ring. In case of TMChM, however, this CE exhibits extreme solvent dependence: it is negative in non-coordinating solvents and positive in coordinating ones, irrespective of the helicity of the heterocyclic ring. TD-DFT calculations using PCM and hybrid solvation models were performed to explain origin of this phenomenon. It turned out that the 1Lb CE sign directly depends on the position of the phenolic OH group at carbon atom C6 (OHC6). In the absence of interactions with solvents (as in CCl4 or nC6H14) or when a solvent plays proton donor role (as in CHCl3), the OHC6 lies in the phenyl plane and the 1Lb CE sign follows the P/M helicity rule. In contrast, in proton acceptor solvents, like DMSO, CH3OH or CH3CN, the OHC6 group is deflected from the phenyl plane, and the 1Lb CE sign of individual (S)-TMChM conformers depends on the sector in which the OHC6 is located. Thus, in solution, the 1Lb CE sign is an average over different orientations of the OHC6 group and can be positive (as in DMSO and CH3OH) or negative (as in CH3CN) which means that it does not follow the chromane helicity rule. The impact of OHC6 on the 1Lb CE sign and thus the conclusions for the stereochemistry of chromans are demonstrated here for the first time. Additionally, a comparison of experimental and simulated ECD spectra, supported by VCD data, allowed to determine the geometry of intermolecular clusters formed in different solvents.
- This article is part of the themed collection: 2018 PCCP HOT Articles


 Please wait while we load your content...
Please wait while we load your content...