Sn–Bi–Sb alloys as anode materials for sodium ion batteries†
Abstract
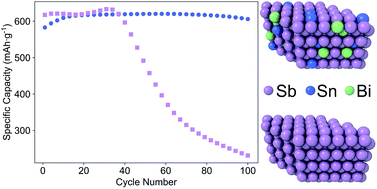
In this work, the performance and electrochemical charge/discharge behavior of Sn–Bi–Sb alloy films were examined, as well as pure Sn, Bi, and Sb films, as anodes for sodium ion batteries (SIBs). Alloying was utilized as an approach to modify the morphology and active phases in an effort to improve the cycling stability of elemental anodes of Sn or Sb, while maintaining a high capacity. The films were prepared via sputtering, which enabled study of a broad swath of compositional space. The cycling performance of the Sb-rich compositions surpassed that of all other alloys tested as anodes for SIBs. The best performing alloy had a composition of 10 at% Sn, 10 at% Bi, and 80 at% Sb (called Sn10Bi10Sb80, here), and maintained 99% of its maximum capacity during cycling (621 mA h g−1) after 100 cycles. Stability of these anodes dropped as the quantity of Sb decreased; to contrast, Sn20Bi20Sb60, Sn25Bi25Sb50 and Sn33Bi33Sb33 were increasingly less stable as anodes in SIBs as the molar quantity of Sb in the films dropped to 60%, 50%, and 33%, respectively. The Sn10Bi10Sb80 electrode was found to possess a single phase as-deposited microstructure of Sn and Bi in substitutional solid solution with the Sb lattice and the sodiation sequence was found to be significantly different from pure Sb. Numerous possible mechanisms for the improvement in capacity retention were discussed, where modification and material response to internal stresses by changes in the Sb chemical potential and solid solution strengthening were found to be the most likely.
- This article is part of the themed collections: Celebrating Excellence in Research: Women of Materials Science and CSC100: Celebrating Canadian Chemistry


 Please wait while we load your content...
Please wait while we load your content...