Bifunctional porous non-precious metal WO2 hexahedral networks as an electrocatalyst for full water splitting†
Abstract
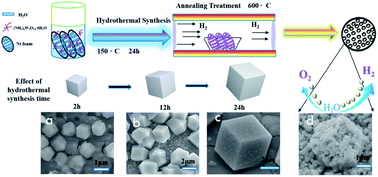
The demand for sustainable hydrogen production by full water splitting has sparked interest in bifunctional non-precious metal-based electrocatalysts with excellent activity and stability for both the hydrogen evolution reaction (HER) and oxygen evolution reaction (OER). Herein, a simple two-step procedure involving hydrothermal synthesis and subsequent annealing treatment was applied to synthesize porous WO2 hexahedral networks supported on nickel foam (WO2 HN/NF). The porous WO2 HN/NF composite reached a current density of −10 mA cm−2 at an overpotential of 48 mV for the HER and 10 mA cm−2 at an overpotential of 300 mV for the OER in an alkaline electrolyte (1.0 M KOH). According to the density functional theory (DFT) calculations, the high catalytic performance of this materials towards the HER could be attributed to the electronic structure of WO2 within the composite. Based on the remarkable electrochemical performance for both the HER and OER, we constructed a symmetric electrochemical water-splitting device. To afford a water-splitting current density of 10 mA cm−2, the alkaline water electrolyzer based on WO2 HN/NF needed a cell voltage of 1.59 V. In addition, this water electrolyzer remained stable over 48 h under continuous operation, which is as good as the performance of commercial Pt/C-based electrolyzers.


 Please wait while we load your content...
Please wait while we load your content...