Synthesis of hollow Pt–Ag nanoparticles by oxygen-assisted acid etching as electrocatalysts for the oxygen reduction reaction†
Abstract
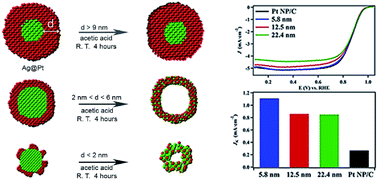
Hollow bimetallic nanostructures have recently shown promising performance for the oxygen reduction reaction (ORR) in fuel cells. In this work, we report the synthesis of hollow Pt–Ag nanoparticles of varying sizes by O2-assisted acid etching of Ag@Pt core@shell nanostructures at room temperature. With a Pt shell less than 6 nm thick, the O2 dissolved in acetic acid could oxidize the Ag core. Subsequently, silver oxide was dissolved in acetic acid and turned into Ag+ ions. During this process, the Ag atoms diffused into the lattice of the Pt shell, and Ag@Pt core@shell nanostructures evolved into hollow Pt–Ag alloy nanoparticles. The as-synthesized hollow Pt–Ag nanocatalysts maintained specific ORR activities that were enhanced beyond the specific activity for commercial carbon supported Pt nanoparticles. The 5.8 nm hollow Pt–Ag nanoparticles displayed the highest activity of 1.12 mA cm−2. Over the course of an accelerated durability test, the 5.8 nm nanoparticles retained 95% and 87% of their initial electrochemical surface area and specific ORR activity. The enhanced activity and durability can be ascribed to the high surface area of the porous structure and the new d-band center due to the hollow morphology and Pt–Ag alloy formation. This work demonstrates a simple strategy for fabricating small porous nanoparticles, which can be potentially used as electrocatalysts in PEM fuel cells.


 Please wait while we load your content...
Please wait while we load your content...