Molecular fractionation of a soil fulvic acid (FA) and competitive sorption of trace metals (Cu, Zn, Cd, Pb) in hematite–solution systems: effect of the FA-to-mineral ratio†
Abstract
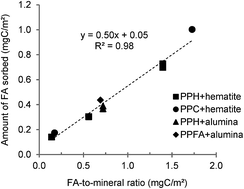
Understanding of the interactions occurring between fulvic acids (FAs) and trace metals in mineral–solution systems is a major issue for cycles of organic matter and micro-pollutants in surface media. Batch experiments and molecular-scale investigations were conducted to address the mechanisms regulating the molecular fractionation of a terrestrial FA and the competition between Cu, Zn, Cd and Pb during sorption onto hematite, a mineral ubiquitous in soils, with a focus on effects of FA-to-mineral ratio (r). A main result is that r controlled both the identity of the FA molecules preferentially sorbed on hematite and the sequence of the FA-promoted sorption of metals, at acidic pH. Data at moderate r evidenced that the sorption degree of a FA molecule increased with molecular acidity, supporting surface complex formation at hematite sites involving preferentially the most acidic and oxygenated FA molecules. FA-promoted sorption of strong Lewis acids such as Pb and Cu was favored (relative to Cd or Zn) by sorbed FA bearing multiple oxygenated functionalities. In contrast, preferential uptake of condensed aromatics and low oxygenated aliphatics/not-condensed aromatics prevailed at high r. A reduced FA-promoted sorption of Cu, which contrasted with an increased FA-promoted sorption of Cd, was observed too. Complex interactions must be invoked (competitive effects, hydrophobic forces, hydrogen bonding) to explain the striking results obtained in highly-competitive FA-concentrated systems. Our data highlight that (coupled) retention/mobilization of reactive organic molecules and of toxic metals like Cd and Pb is a function of the FA-to-metal oxide ratio of soils.


 Please wait while we load your content...
Please wait while we load your content...