Molecular-level insight into the binding of arginine to a zwitterionic Langmuir monolayer†
Abstract
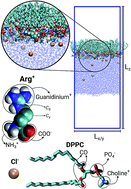
Solutions of the cationic amino-acid arginine (Arg+) in contact with a phospholipid monolayer are investigated by molecular dynamics simulations. The results show that Arg+ binds strongly to the lipid/water interface, with adsorption free-energies ranging from −43.8 to −22.2 kJ mol−1, depending on the amino-acids concentration. The large binding energies are attributed to hydrogen bonding between the charged moieties Arg+ and the phosphate and carbonyl groups of the phospholipids, that compensate for changes in the levels of hydration upon adsorption. We show that a concentrated layer of Arg+, tightly bound to the interface, has little effect on the compression isotherm and the lateral mechanical properties of the monolayer, while having a substantial impact on the interfacial electrostatic potential and the lateral mobility of the lipids. These effects are readily explained in terms of the arrangement that the amino-acids adopt when bound to the monolayer.
- This article is part of the themed collection: Editors' collection: Physical Chemistry of Colloids and Interfaces


 Please wait while we load your content...
Please wait while we load your content...