l-Glutamine-assisted synthesis of flower-like NiO and ball-flower-like NiO/Ag as an electrochemical sensor for lead(ii) detection
Abstract
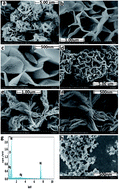
Flower-like NiO (F-NiO) and ball-flower-like NiO/Ag (BF-NiO–Ag) were synthesized in the presence of L-glutamine as an electrochemical sensor for lead(II) detection. The structure and morphology of the F-NiO and BF-NiO–Ag were studied by X-ray diffraction (XRD) and field emission scanning electron microscopy (FESEM). Energy dispersive X-ray confirmed the presence of Ag on the surface of the BF-NiO–Ag. The results confirmed that Ag+ was reduced to Ag metal in the presence of L-glutamine. The FESEM images confirmed the effect of L-glutamine and Ag on the morphology and the available surface area. Electrochemical impedance spectroscopy results confirmed the effect of the loaded Ag on the conductivity of the BF-NiO–Ag. Differential pulse voltammetry results gave a linear working range for the concentration of lead(II) between 1.5 and 10 nM with a LOD of 0.06 nM (S/N = 3). The sensitivity of this linear segment is 0.3 μA nM−1. The average recoveries, recovery percentage and relative standard deviation obtained from four determinations (n = 4) confirmed the feasibility of the proposed method for the quantitative detection of certain concentration ranges of Pb2+.


 Please wait while we load your content...
Please wait while we load your content...