Ezetimibe alleviates non-alcoholic fatty liver disease through the miR-16 inhibiting mTOR/p70S6K1 pathway
Abstract
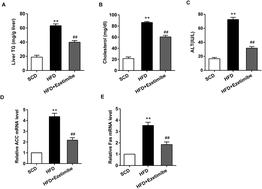
Emerging studies have indicated the role of ezetimibe, miR-16 and mTOR signaling in non-alcoholic fatty liver disease (NAFLD). However, few studies have demonstrated their correlation and regulation functions in NAFLD. Therefore, the protective effect of ezetimibe in NAFLD and the potential mechanisms were explored in the current study. A NAFLD in vivo rat model was established. In addition, a rat hepatocyte cell line, BRL3A, was treated with 1 mM free fatty acids (FFA) to induce NAFLD in vitro. Hepatic triglyceride and lipid metabolism genes (ACC, Fas) and serum biochemical parameters (cholesterol, ALT) were measured. miR-16 and mTOR/p70S6K1 pathway protein expression in both rat livers and cultured cells was also assessed. The direct targeting relationship between miR-16 and mTOR was determined by the dual luciferase reporter gene assay. BRL3A cells were transfected with an miR-16 mimic for in vitro functional studies, and HFD-fed rats were tail vein injected with miR-16 inhibitor and in vivo experimental validation was carried out. NAFLD models both in vivo and in vitro were established successfully. The expression of hepatic triglyceride and lipid metabolism genes was increased and serum biochemical parameters, miR-16 and mTOR/p70S6K1 pathway proteins, were unusually expressed in NAFLD rats and BRL3A cells. Ezetimibe was found to alleviate abnormal expression of these molecules. Through the dual luciferase reporter gene assay, we found that mTOR was a target gene of miR-16. In addition, miR-16 over-expression could reverse the up-regulation of lipid metabolism genes in BRL3A cells, while this effect could be attenuated by pcDNA-mTOR. Ezetimibe significantly reduced the expression of ACC and Fas. After rapamycin treatment, the action of ezetimibe in regulating Fas and AAC was not reversed; however, rapamycin further reduced the expression of ACC and Fas. Silencing miR-16 reversed the protections given by ezetimibe in HFD rats, which showed up-regulated hepatic triglyceride, lipid metabolism genes, serum biochemical parameters and mTOR/p70S6K1 pathway proteins. Ezetimibe alleviated NAFLD induced by a HFD, at least partly, through inhibition of the mTOR/p70S6K1 pathway by miR-16 and affecting the regulation of lipid metabolism, which provides a therapeutic method for NAFLD.


 Please wait while we load your content...
Please wait while we load your content...