Changes in molecular film metallicity with minor modifications of the constitutive quinonoid zwitterions†
Abstract
Molecular films of quinonoid zwitterions, of the general formula C6H2(![[horiz bar, triple dot above]](https://www.rsc.org/images/entities/char_e0f1.gif) O)2(
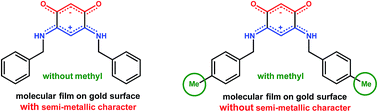
O)2(![[horiz bar, triple dot above]](https://www.rsc.org/images/entities/char_e0f1.gif) NHR)2, have been shown to display electronic properties highly dependent on the nature of the N-substituent R when deposited on gold substrates. The different spacing and organization of the molecules can lead to molecular films with semi-metal or dielectric behavior. With the long term goal to establish how packing effects in the solid state correlate with properties as thin films, we first attempted to identify by X-ray diffraction analysis candidate molecules showing suitable packing arrangements in the crystalline state. To this end, we have prepared a series of new functionalized, enantiopure or sterically-hindered quinonoid zwitterions and established the crystal structure of those with R = CH2–CH2–Ph (6), CH2–CH2–CH2–Ph (7), CH2–CH2–CH2–CH2–Ph (8), CH2–CH2–CH(Ph)–Ph (9), CH(CH3)–Ph (12), CH(CH2–CH3)–Ph (13), CH2–((4–CH3)–C6H4) (15), CH2–((4–NH2)–C6H4) (19) and CH2–CH2–((3,4–(OCH3)2)–C6H3) (24). An analysis of the crystal packing of three molecules, 5, 13 and 15, selected as illustrative examples for comparisons, was carried out and it was unexpectedly found that these chemically very similar molecules gave rise to different packing in the bulk, with resulting thin films showing different electronic properties. Various methods have been used for the characterization of the films, such as synchrotron radiation-based FTIR spatial spectra-microscopy, which provided an anchoring map of zwitterion 15 on a patterned substrate (Au/SiO2) showing its selective anchoring on gold. This is one of the best examples of preferential anchoring of a zwitterion and the sole example of spatial localization for a quinonoid zwitterion thin film. We have also used combined photoemission and inverse photoemission spectra and the data were compared to occupied and unoccupied DFT density state calculations.
NHR)2, have been shown to display electronic properties highly dependent on the nature of the N-substituent R when deposited on gold substrates. The different spacing and organization of the molecules can lead to molecular films with semi-metal or dielectric behavior. With the long term goal to establish how packing effects in the solid state correlate with properties as thin films, we first attempted to identify by X-ray diffraction analysis candidate molecules showing suitable packing arrangements in the crystalline state. To this end, we have prepared a series of new functionalized, enantiopure or sterically-hindered quinonoid zwitterions and established the crystal structure of those with R = CH2–CH2–Ph (6), CH2–CH2–CH2–Ph (7), CH2–CH2–CH2–CH2–Ph (8), CH2–CH2–CH(Ph)–Ph (9), CH(CH3)–Ph (12), CH(CH2–CH3)–Ph (13), CH2–((4–CH3)–C6H4) (15), CH2–((4–NH2)–C6H4) (19) and CH2–CH2–((3,4–(OCH3)2)–C6H3) (24). An analysis of the crystal packing of three molecules, 5, 13 and 15, selected as illustrative examples for comparisons, was carried out and it was unexpectedly found that these chemically very similar molecules gave rise to different packing in the bulk, with resulting thin films showing different electronic properties. Various methods have been used for the characterization of the films, such as synchrotron radiation-based FTIR spatial spectra-microscopy, which provided an anchoring map of zwitterion 15 on a patterned substrate (Au/SiO2) showing its selective anchoring on gold. This is one of the best examples of preferential anchoring of a zwitterion and the sole example of spatial localization for a quinonoid zwitterion thin film. We have also used combined photoemission and inverse photoemission spectra and the data were compared to occupied and unoccupied DFT density state calculations.
- This article is part of the themed collection: 1st International Conference on Noncovalent Interactions


 Please wait while we load your content...
Please wait while we load your content...