Direct C(sp2)–H amination of aryl aldehyde-derived hydrazones via visible light promoted photoredox catalysis†
Abstract
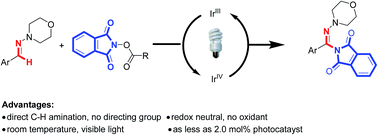
An unprecedented visible-light-induced direct C(sp2)–H amination of aryl aldehyde-derived hydrazones was developed by using N-acyloxyphthalimides as nitrogen-radical precursors. This reaction represents a new way to substituted hydrazones with amination of carbon–nitrogen π bonds. A radical C–H amination mechanism is proposed.


 Please wait while we load your content...
Please wait while we load your content...