Oxygen permeation, thermal expansion behavior and electrochemical properties of LaBa0.5Sr0.5Co2O5+δ cathode for SOFCs†
Abstract
In this study, the double perovskite LaBa0.5Sr0.5Co2O5+δ (LBSC55) is investigated as a potential cathode for solid oxide fuel cells (SOFCs). In the curve of the first derivative δL vs. temperature, there is an inflection region ranging from 230 to 330 °C. The peak inflection point located about 260 °C is associated with the initial temperature for the loss of lattice oxygen and the formation of oxygen vacancies. The concentration of Co4+ ions in LBSC55 decreased significantly above 260 °C due to the loss of lattice oxygen (Co4+ → Co3+) and creation of 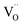 at the same time. The electrical conductivity exhibited a metallic behavior with values that gradually decreased from 232 S cm−1 at 400 °C to 178 S cm−1 at 800 °C. The chemical bulk diffusion coefficient (Dchem) values of LBSC55 are 1.49 × 10−4, 2.75 × 10−4 and 5.37 × 10−4 cm2 s−1 at 700 °C, 750 °C and 800 °C, respectively. The oxygen permeation flux for LBSC55 membrane with 1.0 mm thickness increased from 0.210 mL min−1 cm−2 at 500 °C to 0.302 mL min−1 cm−2 at 800 °C under synthetic air at a flow rate of 50 mL min−1, with helium at a rate of 25 mL min−1. The activation energies of oxygen permeation for the high temperature region (750–800 °C) and low temperature region (500–700 °C) are 10.60 and 2.75 kJ mol−1, respectively. This suggested that the surface exchange process is dominant above 750 °C and at low temperature ranges the bulk diffusion process is dominant for oxygen ion diffusion. The main process of the oxygen reduction reaction is dominated by oxygen ion transfer from TPB and/or 2PB sites of the cathode to electrolyte in the temperature range of 600–800 °C. Based on the chemical bulk diffusion coefficient, oxygen permeation and electrochemical properties, LBSC55 is a potential cathode for solid oxide fuel cells (SOFCs).
at the same time. The electrical conductivity exhibited a metallic behavior with values that gradually decreased from 232 S cm−1 at 400 °C to 178 S cm−1 at 800 °C. The chemical bulk diffusion coefficient (Dchem) values of LBSC55 are 1.49 × 10−4, 2.75 × 10−4 and 5.37 × 10−4 cm2 s−1 at 700 °C, 750 °C and 800 °C, respectively. The oxygen permeation flux for LBSC55 membrane with 1.0 mm thickness increased from 0.210 mL min−1 cm−2 at 500 °C to 0.302 mL min−1 cm−2 at 800 °C under synthetic air at a flow rate of 50 mL min−1, with helium at a rate of 25 mL min−1. The activation energies of oxygen permeation for the high temperature region (750–800 °C) and low temperature region (500–700 °C) are 10.60 and 2.75 kJ mol−1, respectively. This suggested that the surface exchange process is dominant above 750 °C and at low temperature ranges the bulk diffusion process is dominant for oxygen ion diffusion. The main process of the oxygen reduction reaction is dominated by oxygen ion transfer from TPB and/or 2PB sites of the cathode to electrolyte in the temperature range of 600–800 °C. Based on the chemical bulk diffusion coefficient, oxygen permeation and electrochemical properties, LBSC55 is a potential cathode for solid oxide fuel cells (SOFCs).



 Please wait while we load your content...
Please wait while we load your content...